The ampoule break strength testing machine plays a pivotal role in ensuring the quality and safety of parenteral drugs packaging. This testing is vital for evaluating the strength and integrity of ampoules used in medical applications, where the ability to break cleanly and safely is crucial. In this article, we will explore the importance of ampoule break force testing, the role of glass ampoule break force testers, and the relevant standards, including ISO 9187-1.
Parenteral Drugs Packaging: Ensuring Safety and Efficacy
The packaging of parenteral drugs is a critical element in the pharmaceutical industry. These drugs, typically administered via injection, require robust and secure packaging to prevent contamination and ensure that the drug can be delivered safely to patients. Ampoules, as single-use containers for injectable medications, must meet stringent requirements to guarantee they can withstand transportation, handling, and provide an effective, safe break when opened.
Testing the ampoule break strength is essential to ensure the packaging is not only durable but also designed for easy, controlled opening during use. The ampoule break strength testing machine evaluates the amount of force required to break the ampoule at the intended breaking point, which is often designed with a predetermined area for breakage, such as a ceramic ring.
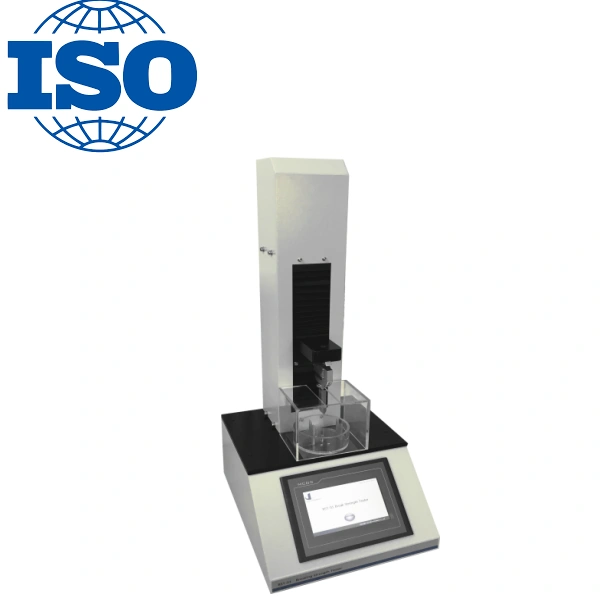
Glass Ampoule Break Force Tester: A Critical Tool
A glass ampoule break force tester is designed to measure the breaking strength of ampoules under controlled conditions. This tester applies a precise force to the ampoule to ensure it breaks cleanly without creating sharp edges or posing any risks during the use of injectable drugs. The test involves subjecting the ampoule to a force until it breaks at the predetermined break point.
The ampoule break force is measured to ensure that the ampoule will open with minimal force, ensuring safety for healthcare professionals. It also guarantees that the packaging can withstand standard handling during transport and storage without breaking prematurely.
Importance of ISO 9187-1 in Ampoule Testing
The ISO 9187-1 standard, titled Injection Equipment for Medical Use — Part 1: Ampoules for Injectables, outlines the requirements for testing the breaking force of ampoules. According to this standard:
- Annealing quality: Ampoules must be annealed to prevent excessive residual stress, which could impact their breaking strength.
- Hydrolytic resistance: Ampoules must meet hydrolytic resistance standards to ensure that the internal surface of the ampoule does not degrade or react with the medication.
- Breaking force testing: The breaking force test determines whether the force required to separate the ampoule’s stem from its body is within specified limits. This ensures that the break is clean and controlled, reducing the risk of shards and contamination.
The ISO 9187-1 standard specifies the test setup, equipment, and the required breaking force for different types of ampoules, ensuring that they perform consistently in various conditions.
Procedure for Testing Ampoule Break Strength
The process for testing the ampoule break strength typically follows these steps:
- Sample Preparation: Ampoules are selected and conditioned at a temperature of 20°C ± 5°C, as recommended by the standard. This ensures consistent testing conditions.
- Test Setup: The ampoule is placed in a tensile testing machine, and the force is applied at a speed of 10 mm/min. The testing setup must ensure that the force is applied to the middle of the ampoule stem at a 90° angle to the axis.
- Force Application: The tester applies force until the ampoule breaks. The breaking force is recorded to determine if it meets the required strength for safe and controlled opening.
- Result Analysis: The results are compared against the specified limits from ISO 9187-1. If the force required to break the ampoule falls within the acceptable range, the ampoule is considered to have met the quality requirements.
Ampoule Bending Tester: Complementary Testing for Ampoules
In addition to the ampoule break force tester, an ampoule bending tester is used to evaluate the flexibility and resistance to bending of ampoules. This test is particularly useful for assessing the overall durability of ampoules during handling. The ampoule bending tester applies a bending force to the ampoule to simulate real-world handling and transportation conditions. This test complements the break strength test by ensuring that the ampoule can withstand bending forces without cracking or breaking before it reaches the point of intended breakage.
Conclusion: Reliable Packaging for Parenteral Drugs
The ampoule break strength testing machine is essential for ensuring the quality and safety of parenteral drugs packaging. By accurately testing the ampoule break force, manufacturers can ensure that their ampoules will perform as expected under real-world conditions, providing a controlled and clean break when needed.
At Cell Instruments, we offer advanced testing solutions, including the BST-01 Ampoule Breaking Tester, to help you ensure the highest quality for your parenteral drugs packaging. Regular testing and adherence to standards like ISO 9187-1 are crucial to maintaining the integrity and safety of pharmaceutical packaging.